A Brief Explanation of Evaporative Cooling
To ensure a truly comprehensive range of cooling equipment we carry a wide selection of Evaporative Coolers which are ideally suited for cooling areas from 120 ft² (11.1 m²) to large areas in excess of 2000 ft² (186 m²). These machines can be “zones” for use in specific areas and simply require a supply of water and electrical power.
One of the major differences between an Evaporative Air Cooler and either a Portable or fitted-system is that evaporative coolers do not have exhaust hoses or outside condenser boxes. They are also different from Air Conditioners in that they do not provide “refrigerant air” but rather provide “cooled air”. To give you an example of the principle used in evaporative cooling simply try the following experiment. On a warm summer day place your arm in a bucket of warm water and then remove it. You will feel a slight chill over the wet area. This has been caused by the evaporation of the water off your arm; the very action of evaporation is the “energy” for the cooling. Whilst very powerful and suitable for many applications, evaporative cooling is inexpensive both to purchase and to run. It is more effective in drier climates and can be used for everything from residential homes to large buildings such as warehouses and shops etc.
Evaporative coolers are a very effective, economical and environmentally friendly method of cooling. It is important to note, however, that there are restrictions as to the appropriate uses for Evaporative Coolers. For example, they must not be used in a closed and unventilated area because they rely on a flow of fresh air to enable the process to work. Having been filled with water, if they are used in an unventilated area they will simply pump humidity into that area making it very damp and this could be damaging to electrical systems or contents in that area.
Therefore Evaporative Air Conditioning is suitable for areas with adequate natural ventilation and not “sealed” spaces. Although there are many Evaporative Coolers in use, very often the users are not really familiar with the actual principles of how evaporative cooling works. A basic knowledge of these principles will avoid problems occurring and improve the cooling ability of these excellent systems.
What is Evaporative Cooling?
To help understand the principles of Evaporative Cooling it’s a good idea to think of the air as a type of sponge. As air comes into contact with water, it absorbs it and becomes damp air or humid air. The amount of water that the air can absorb depends largely on how much water is already in the air. Obviously, a dry sponge will absorb more spillage than a wet sponge. Generally, the term “humidity” is used to describe the amount of water in the air. For instance, if in a mixture of air and water 20% is water, then we state that the humidity would be 20%. If the humidity is 100% that would indicate that the air is holding all the moisture it can and it is probably raining or extremely damp.
Obviously the lower the humidity the more room there is in the air for more water and a larger amount of evaporation can take place. Remember the action of evaporation is the cooling power behind Evaporative Coolers. We use the term “relative humidity” when describing the amount of moisture in the air. The “sponginess” of the air changes according to the air’s temperature. If the air is warm it becomes more spongy and can therefore hold more water. Because of this we must describe the level of humidity as relative to the temperature because this affects the ‘sponginess’ or absorbability of the air. Therefore, a room at 50% humidity will hold more water at 80°F than at 50°F.
How is the Cooling Produced
Energy in the form of heat is required to evaporate water. Actually, to evaporate a gallon of water requires 8,700 BTUs (British Thermal Units) of heat. The heat comes from whatever the water is in contact with which could be a building, your body, a tree or the heat of the air itself. As the heat comes into contact with the water the temperature the heat-source is reduced.
An important point to understand is that the temperature of the water does not have any real effect on the cooling produced by the evaporation process. If you were to throw a gallon of water at 50°F on a warm pavement of say 90°F, it would produce 9,000 BTUs of cooling. If you were to throw a gallon of 90°F water onto the pavement it would produce 8,700 BTUs of cooling which is only a 3% difference. Imagine being sprayed with water at either of those temperatures on a hot day; as it evaporated off your skin you would feel much cooler.
The construction of an evaporative cooler is such that filter pads are located at the back or sides (or both) of the machine and the surrounding warm air is sucked into the machine through the pads. The pads are kept damp either by water being pumped from an internal reservoir around and through the pads or from an external water source. As the outside warm air moves across the wet pad the water is evaporated, removing the heat from the air. Therefore when that air exits the machine it is in a cooler state than when it entered the machine.
The best Evaporative Coolers are able to provide the largest surface area over which the air can travel and evaporate the water. It is important, therefore, for the machine to draw in as much warm air as it can and pass it over as large a “damp” area as possible.
Some practical notes:
• The lower the relative humidity the better the performance of the cooler. Typically 70% are RH or below is good.
• Cold or iced water does not make much difference to the cooling efficiency of an Evaporative Cooler.
• Evaporative Coolers must only be used in areas with good natural ventilation or adequate mechanical air extraction units.
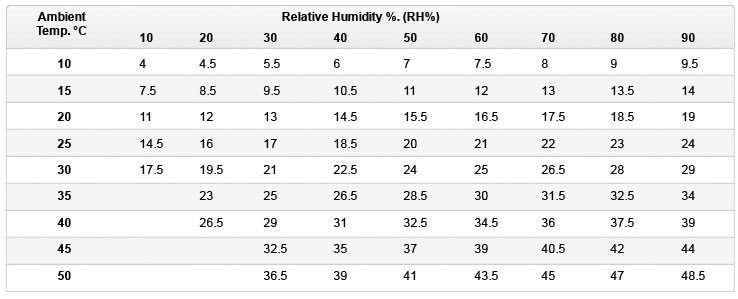
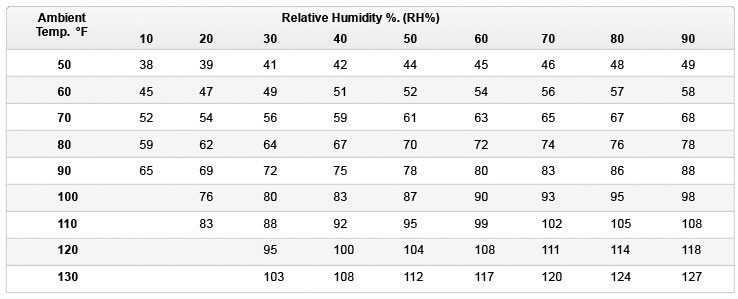
Evaporative Air Cooler Temperature Drop Charts
The charts below show the approximate temperature of the cooled air as it leaves the front of the evaporative cooler given the ambient temperature and relative humidity (RH) combination. The figures assume that the cooling pads are 80% saturated. The first chart shows °C (Centigrade) the second °F (Fahrenheit).